The Geometry of the Clf3 Molecule Is Best Described as. Cl 1 s 2 2 s 2 2 p 6 3 s 2 3 p 5.
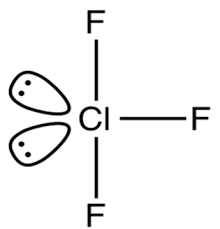
Clf3 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape
The electrophilic aromatic substitution reaction can be described as the type of chemical reaction i.

. The shape of ClF3 molecule is best described as. F2o Or Of2 Lewis Dot Structure Molecular Shape Electron Geometry. Orbital hybridization is an essential concept of chemical bonding that we cannot skip.
What is the formal charge of oxygen in N2O-1. Chlorine trifluoride has 10 electrons around the central chlorine atom. ClF 3 molecule has a trigonal bipyramidal T-shaped shape.
The Shape of ClF3 Molecule Is. Chlorine atom has seven valence electrons. In a ClF5 molecule chlorine is the central atom featuring five single bonds with fluorine atoms.
Answer the questions in the table below about the shape of the chlorine trifluoride CIF3 molecule. The shape of H2O molecule is angular and its hybridisation is sp3 whereas the shape of BeF2 molecule. Expected shape is Trigonal biPyramidal but dueto the presence of TWO lone pairs of electrons the shape is T shaped But actually.
The lewis dot structure for AsCl3 shows. Asked Dec 19 2017 in Chemistry by sforrest072 128k points p - block element. To learn more chemistry-related question and answers visit BYJUS The Learning App.
The VSEPR theory suggests that the molecular shape of ClF3 is T- shaped. There are two equatorial lone pairs making the final structure T shaped. The electronic configuration of chlorine atom is as below.
Solution for What is the shape of ClF3. Chlorine trifluoride molecule has three Cl F bonds and two lone pairs of electrons on central atom Chlorine. Correct Option - C T-shaped.
What is the geometry of PO4 3-Tetrahedral. This creates five regions of electron density around the central chlorine atom. Steric number in ClF3 3 No.
ClF3 molecule has a bent T-shaped structure and not a trigonal planar structure. Now have a look at the above chart again. The shape of the molecule ClF3 is-.
Of bonded electrons 2 Lone pair 5. A distorted tetrahedron D T-shaped B trigonal planar E trigonal pyramidal C tetrahedral. First week only 499.
Since chlorine has 7 electrons in the valence shell the remaining two electrons form a lone pair. ClF3 molecule possesses trigonal bipyramidal molecular geometry. The shape of ClF3 molecule is T shaped The hybridisation is Sp3d.
The geometry of the ClF3 molecule is best described as. How many electron groups are around the central chlorine atom. ClF3 Hybridization What is Orbital Hybridization.
April 11 2022 Ocl2 Lewis Structure Dichlorine Monoxide Molecules Lewis Electrons Clf3 Molecular Geometry Bond Angles Electron Geometry Molecular Geometry Molecular Molecules. As per the VSEPR theory valence shell electron pair repulsion theory the shape of a ClF5 molecule is square pyramidal. Three single bonds and 10 lone pairs.
One electron group means one lone pair one single bond one double bond or one triple bond. Start your trial now. What is the shape of the SO3 molecule.
The shape of the ClF3molecule is T-shaped. In Cl F 3 the chlorine is a central atom. Because the center atom chlorine has three Cl-F bonds with the three fluorine atoms surrounding it.
A correct answer is an option C T-Shape. In Cl F 3 two lone pairs are placed in axial plane which minimises the repulsion and stabilises the molecule. This means there are five electron pairs arranged in a trigonal bipyramidal shape with a 175 F C l F bond angle.
What phrase best describes the arrangement of these electron groups.
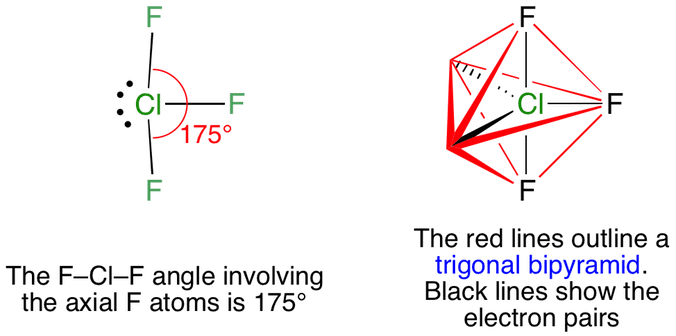
Vsepr Clf3 Chlorine Trifluoride

Clf3 Molecular Geometry Bond Angles Electron Geometry Chlorine Trifluoride Youtube
What Is The Molecular Geometry Of Cif3 Ci Not Cl Quora

Hybridization Of Clf3 Hybridization Of Cl In Chlorine Trifluoride
0 Comments